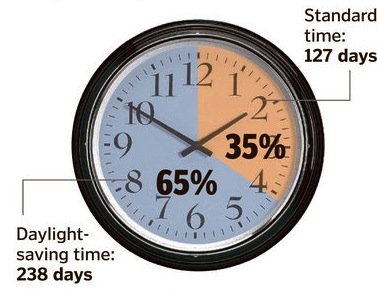
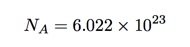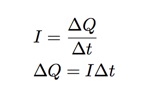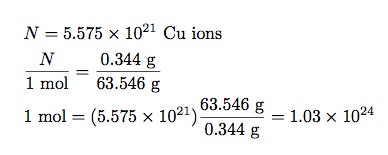A veteran is someone who, at one point in his life, wrote a blank check made payable to his country for an amount of ‘up to and including my life.’
- Andy
- Beef on Weck
- Being a Mother
- Chili Cook-off
- Communication (Gazebo)
- Daddy
- Everyone has an Angel
- Family
- Gonna be a Bear
- Harrison Bergeron
- Mute and Alone
- Privacy Policy
- Rikki-tikki-tavi
- Scientists Study Grizzly Bears
- Ship vs. Lighthouse
- Snowvember (Buffalo 2014)
- Somebody…
- The Present
- The Soldier
- The Star
- Winter
- 11foot8.com
- 365 Tomorrows
- 7 into 28
- A Tale of Two Brains
- Alien to Covenant – History of Alien
- Am I Unique
- AMARC
- American Muscle Car Museum
- Andre Rieu
- Antipodes Map
- Ark in Space
- Azure Status
- Blizzard of '77
- Broken Chains
- CDC – Flu
- Christmas Forever AZ
- Coldest City on Earth
- Creations for Charity (Lego)
- Cruise.com
- Curb Watching
- D&D Beyond
- D&D Beyond to FG Character Converter
- Daily Fuel Gauge Report
- Dinosaur Earth
- DMs Guild
- Dofo
- Dr. Demento
- DriveThru RPG
- Dungeon in a Box
- Dyson’s Dodecahedron
- Fantasy Name Generator
- Farmer's Donkey
- Fast Character
- Flight Aware
- Flight Radar 24
- Flixable
- Gaming Table
- Genius
- Geo Guesser!
- Hack The Menu
- Hackers for Charity
- Hadzy
- Have I been Pwned
- HexRoll
- How to remove a tick (properly)
- Identity Theft Resource Center
- Leak Lookup
- Line Rider – Hall of the Mountain King
- Make My Drive Fun
- Mapologies
- Marine Traffic
- MathPapa
- MechWarrior Online
- Medieval Murder Maps
- Meteor Shower Calendar
- Mini Building Materials
- Monterey Bay Aquarium
- MyAbandonware
- Nah! I just might be in there!
- National Do Not Call Registry
- No More Ransom
- NOAA – Louisville
- Nobody Live
- Norse Cyber Attack Map
- OCEARCH.org
- Omega Game Shrine
- Out of the Woods Forestry
- Overt
- PC Gaming Wiki
- Percheron
- Periodic Stats
- Periodic Videos (TED)
- Permethin Fact Sheet
- Pigeon Key Foundation
- Project 44
- pTable
- Pumpkin Pile
- Random Restaurant Generator
- Rankin/Bass – Wikipedia
- ReelGood
- RockAuto
- Roll20 Enhancement Suite
- Schimpff's
- Scuba Shooters
- Sinking of the Titanic
- Smoky Mountain Fall Foliage Map
- Speedsums
- SR-71 Speed Check
- Steam Status
- Still Tasty
- StreamSquid
- Sunken Ships of the Second World War
- Super Slice!
- Swedish Fish
- Tank America
- Taste Dive
- TBSP (TaBleSPoon)
- The Louvre
- The Oz Museum
- The Strong National Museum of Play
- They Can Talk
- This Beat Goes on/Switchin' to Glide
- Tick Removal (CDC)
- Trappistine Candy
- Vacation Rentals By Owner
- Vehicle Privacy Report
- VPNFilter Check
- War Puppets Rise to Heaven
- Weather Back Home
- WebGL Water
- Whalers on the Moon
- What's New on Netflix
- Who's On First
- Why are Jacks called Jacks?
- Wild Spirit
- Window Swap
- WKRP Turkey Drop
- Wordcount
- World's Hottest Chocolate Bar
- WWII Portraits of Honor
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011