- Andy
- Beef on Weck
- Being a Mother
- Chili Cook-off
- Communication (Gazebo)
- Daddy
- Everyone has an Angel
- Family
- Gonna be a Bear
- Harrison Bergeron
- Mute and Alone
- Privacy Policy
- Rikki-tikki-tavi
- Scientists Study Grizzly Bears
- Ship vs. Lighthouse
- Snowvember (Buffalo 2014)
- Somebody…
- The Present
- The Soldier
- The Star
- Winter
- 11foot8.com
- 365 Tomorrows
- 7 into 28
- A Tale of Two Brains
- Alien to Covenant – History of Alien
- Am I Unique
- AMARC
- American Muscle Car Museum
- Andre Rieu
- Antipodes Map
- Ark in Space
- Azure Status
- Blizzard of '77
- Broken Chains
- CDC – Flu
- Christmas Forever AZ
- Coldest City on Earth
- Creations for Charity (Lego)
- Cruise.com
- Curb Watching
- D&D Beyond
- D&D Beyond to FG Character Converter
- Daily Fuel Gauge Report
- Dinosaur Earth
- DMs Guild
- Dofo
- Dr. Demento
- DriveThru RPG
- Dungeon in a Box
- Dyson’s Dodecahedron
- Fantasy Name Generator
- Farmer's Donkey
- Fast Character
- Flight Aware
- Flight Radar 24
- Flixable
- Gaming Table
- Genius
- Geo Guesser!
- Hack The Menu
- Hackers for Charity
- Hadzy
- Have I been Pwned
- HexRoll
- How to remove a tick (properly)
- Identity Theft Resource Center
- Leak Lookup
- Line Rider – Hall of the Mountain King
- Make My Drive Fun
- Mapologies
- Marine Traffic
- MathPapa
- MechWarrior Online
- Medieval Murder Maps
- Meteor Shower Calendar
- Mini Building Materials
- Monterey Bay Aquarium
- MyAbandonware
- Nah! I just might be in there!
- National Do Not Call Registry
- No More Ransom
- NOAA – Louisville
- Nobody Live
- Norse Cyber Attack Map
- OCEARCH.org
- Omega Game Shrine
- Out of the Woods Forestry
- Overt
- PC Gaming Wiki
- Percheron
- Periodic Stats
- Periodic Videos (TED)
- Permethin Fact Sheet
- Pigeon Key Foundation
- Project 44
- pTable
- Pumpkin Pile
- Random Restaurant Generator
- Rankin/Bass – Wikipedia
- ReelGood
- RockAuto
- Roll20 Enhancement Suite
- Schimpff's
- Scuba Shooters
- Sinking of the Titanic
- Smoky Mountain Fall Foliage Map
- Speedsums
- SR-71 Speed Check
- Steam Status
- Still Tasty
- StreamSquid
- Sunken Ships of the Second World War
- Super Slice!
- Swedish Fish
- Tank America
- Taste Dive
- TBSP (TaBleSPoon)
- The Louvre
- The Oz Museum
- The Strong National Museum of Play
- They Can Talk
- This Beat Goes on/Switchin' to Glide
- Tick Removal (CDC)
- Trappistine Candy
- Vacation Rentals By Owner
- Vehicle Privacy Report
- VPNFilter Check
- War Puppets Rise to Heaven
- Weather Back Home
- WebGL Water
- Whalers on the Moon
- What's New on Netflix
- Who's On First
- Why are Jacks called Jacks?
- Wild Spirit
- Window Swap
- WKRP Turkey Drop
- Wordcount
- World's Hottest Chocolate Bar
- WWII Portraits of Honor
- April 2025
- March 2025
- February 2025
- January 2025
- December 2024
- November 2024
- October 2024
- September 2024
- August 2024
- July 2024
- June 2024
- May 2024
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- July 2023
- June 2023
- May 2023
- April 2023
- March 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- July 2022
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
- April 2020
- March 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- July 2019
- June 2019
- May 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- November 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- May 2018
- April 2018
- March 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- June 2017
- May 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- May 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- December 2014
- November 2014
- October 2014
- September 2014
- August 2014
- July 2014
- June 2014
- May 2014
- April 2014
- March 2014
- February 2014
- January 2014
- December 2013
- November 2013
- October 2013
- September 2013
- August 2013
- July 2013
- June 2013
- May 2013
- April 2013
- March 2013
- February 2013
- January 2013
- December 2012
- November 2012
- October 2012
- September 2012
- August 2012
- July 2012
- June 2012
- May 2012
- April 2012
- March 2012
- February 2012
- January 2012
- December 2011
- November 2011
- October 2011
- September 2011
- August 2011
- July 2011
- June 2011
- May 2011
- April 2011
Author Archives: James
Dungeons & Dragons turns 50!
Raise a twenty-sided die and Play Your Way!
“D&D has a rich history, an exciting present, and a great future,” said Kyle Brink, Executive Producer of the team making D&D at Wizards of the Coast. “This year we’ll be celebrating all three with the 50th Anniversary of the first publication of Dungeons & Dragons. We’ll take you through the making of the game, bring some of the classic adventures to today’s play, visit the most iconic settings in the D&D multiverse, and kick off the future of the game with the new 2024 core rulebooks that are the heart of the game. We’ve been building up to this for a while now. It’s going to be a lot of fun.”
Read the whole release over on Hasbro’s Release Page!
Posted in Because I Can, Gaming
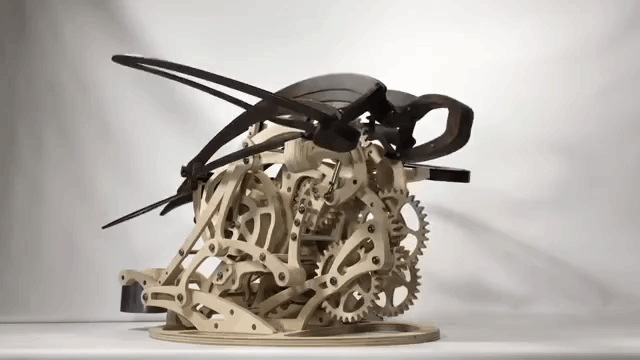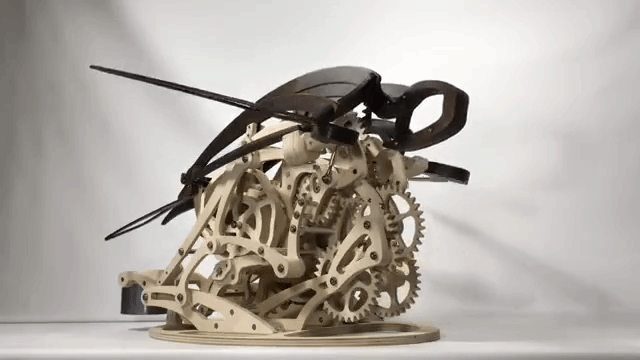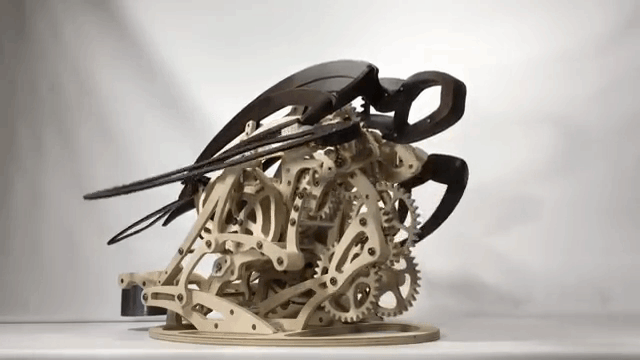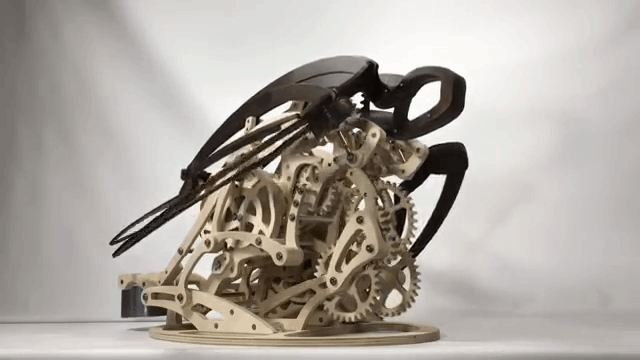
Carapace
Derek Hugger has created another wooden kinetic sculpture simulating the motion of a swimming sea turtle. It is called “Carapace“:

Posted in Gadgets
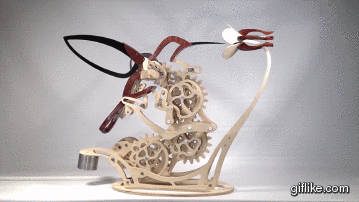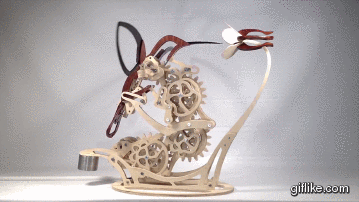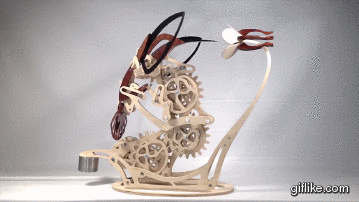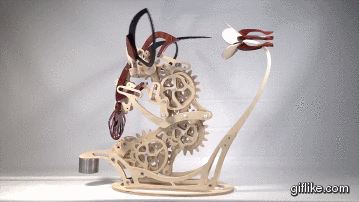
Colibri

Motion artist Derek Hugger has created “Colibri“, a breathtaking hand-cranked kinetic sculpture that painstakingly captures the delicate motions of a hummingbird as it feeds at a flower.
Every element of motion has been completely mechanized, from the beating wings to the flaring tail. Intricate systems of linkages and cams bring the sculpture to life with a continuous flow of meticulously timed articulations. As each mechanism has been linked to the next, Colibri cycles through its complete range of motions by the simple turn of a crank.This project is my eighth woodworking design project and is by far the most complex project I’ve done so far. From start to finish, Colibri took me about 700 hours.
Posted in Gadgets
















